Medical Device Risk Management . learn the basic terms, concepts and methods of risk management for medical devices from the us food and drug. learn how to perform risk management for medical devices according to iso 14971, the international standard for product safety. This guide covers the definition, importance, principles, and requirements of risk management for medical devices. this document provides guidance on the development, implementation and maintenance of a risk management. learn the foundational knowledge of risk management for medical devices from the fda. this document specifies terminology, principles and a process for risk management of medical devices,. This transcript covers basic terms,. learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and.
from qbd.eu
This transcript covers basic terms,. This guide covers the definition, importance, principles, and requirements of risk management for medical devices. this document provides guidance on the development, implementation and maintenance of a risk management. learn the basic terms, concepts and methods of risk management for medical devices from the us food and drug. learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and. learn how to perform risk management for medical devices according to iso 14971, the international standard for product safety. learn the foundational knowledge of risk management for medical devices from the fda. this document specifies terminology, principles and a process for risk management of medical devices,.
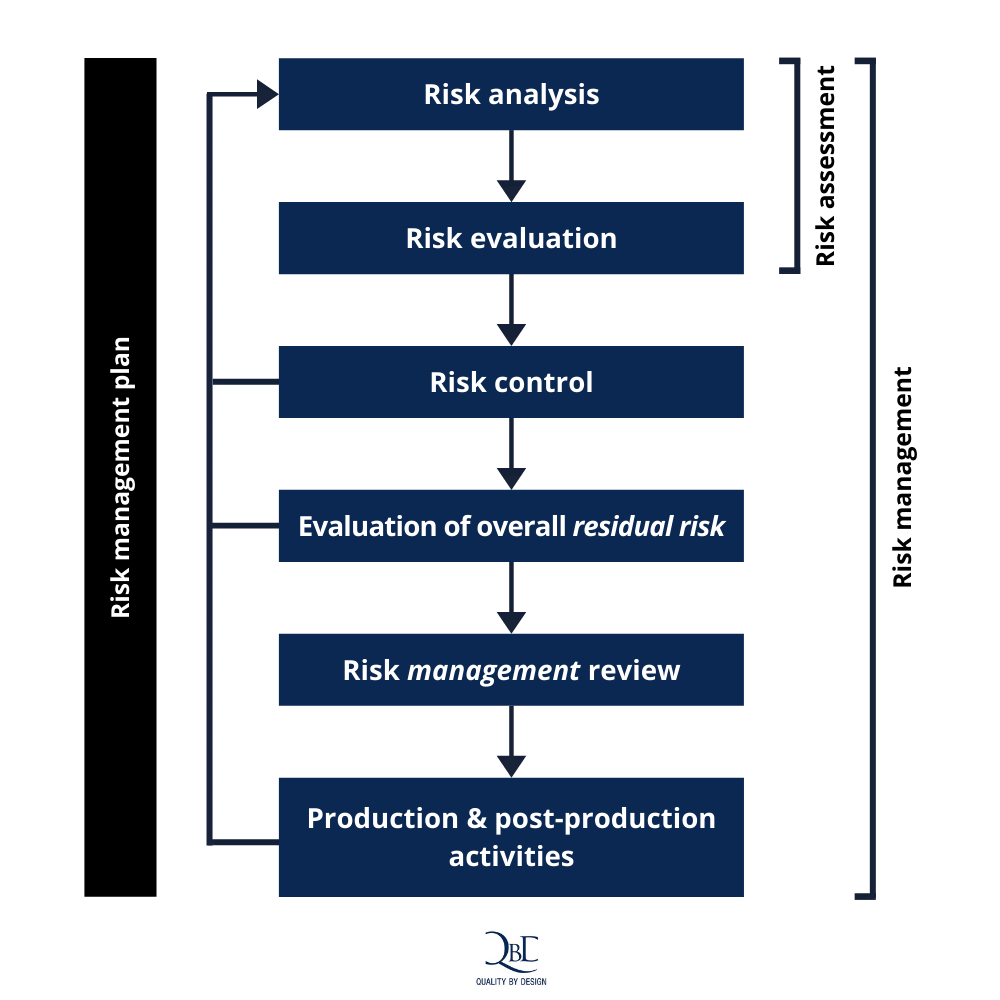
Why Medical Device Risk Management is as complex as it is crucial
Medical Device Risk Management learn the foundational knowledge of risk management for medical devices from the fda. this document specifies terminology, principles and a process for risk management of medical devices,. learn how to perform risk management for medical devices according to iso 14971, the international standard for product safety. this document provides guidance on the development, implementation and maintenance of a risk management. learn the foundational knowledge of risk management for medical devices from the fda. This guide covers the definition, importance, principles, and requirements of risk management for medical devices. learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and. This transcript covers basic terms,. learn the basic terms, concepts and methods of risk management for medical devices from the us food and drug.
From www.orielstat.com
Choosing the right medical device risk management tools Medical Device Risk Management learn the foundational knowledge of risk management for medical devices from the fda. learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and. This guide covers the definition, importance, principles, and requirements of risk management for medical devices. this document provides guidance on the development, implementation and. Medical Device Risk Management.
From exoaernau.blob.core.windows.net
Medical Device Risk Management Documents at Terrence Knapp blog Medical Device Risk Management learn how to perform risk management for medical devices according to iso 14971, the international standard for product safety. This guide covers the definition, importance, principles, and requirements of risk management for medical devices. learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and. This transcript covers basic. Medical Device Risk Management.
From medicaldevicehq.com
Risk management for medical device and ISO 149712019 infographic Medical Device Risk Management This transcript covers basic terms,. this document provides guidance on the development, implementation and maintenance of a risk management. learn how to perform risk management for medical devices according to iso 14971, the international standard for product safety. This guide covers the definition, importance, principles, and requirements of risk management for medical devices. this document specifies terminology,. Medical Device Risk Management.
From qbd.eu
Why Medical Device Risk Management is as complex as it is crucial Medical Device Risk Management This guide covers the definition, importance, principles, and requirements of risk management for medical devices. this document provides guidance on the development, implementation and maintenance of a risk management. learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and. this document specifies terminology, principles and a process. Medical Device Risk Management.
From corsi-dm.it
Introduction to Medical Device Safety Risk Management in Compliance Medical Device Risk Management learn the foundational knowledge of risk management for medical devices from the fda. This transcript covers basic terms,. this document provides guidance on the development, implementation and maintenance of a risk management. this document specifies terminology, principles and a process for risk management of medical devices,. This guide covers the definition, importance, principles, and requirements of risk. Medical Device Risk Management.
From studylib.net
Medical Device Risk Management Medical Device Risk Management learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and. learn the foundational knowledge of risk management for medical devices from the fda. This transcript covers basic terms,. learn how to perform risk management for medical devices according to iso 14971, the international standard for product safety.. Medical Device Risk Management.
From sunstonepilot.com
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc. Medical Device Risk Management learn the foundational knowledge of risk management for medical devices from the fda. learn the basic terms, concepts and methods of risk management for medical devices from the us food and drug. learn how to perform risk management for medical devices according to iso 14971, the international standard for product safety. This transcript covers basic terms,. . Medical Device Risk Management.
From mavenprofserv.com
Medical Device Risk Management Assessing Overall Residual Risk Medical Device Risk Management learn the foundational knowledge of risk management for medical devices from the fda. learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and. learn how to perform risk management for medical devices according to iso 14971, the international standard for product safety. this document specifies terminology,. Medical Device Risk Management.
From shotnelo.weebly.com
Medical device risk assessment template shotnelo Medical Device Risk Management learn the foundational knowledge of risk management for medical devices from the fda. learn the basic terms, concepts and methods of risk management for medical devices from the us food and drug. this document specifies terminology, principles and a process for risk management of medical devices,. This transcript covers basic terms,. learn how to perform risk. Medical Device Risk Management.
From www.orielstat.com
Preparing a Medical Device Risk Management Review and Report Medical Device Risk Management learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and. this document provides guidance on the development, implementation and maintenance of a risk management. This guide covers the definition, importance, principles, and requirements of risk management for medical devices. this document specifies terminology, principles and a process. Medical Device Risk Management.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis Medical Device Risk Management learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and. learn the basic terms, concepts and methods of risk management for medical devices from the us food and drug. this document specifies terminology, principles and a process for risk management of medical devices,. learn the foundational. Medical Device Risk Management.
From medicaldevicehq.com
Risk management for medical device and ISO 149712019 infographic Medical Device Risk Management learn the foundational knowledge of risk management for medical devices from the fda. learn how to perform risk management for medical devices according to iso 14971, the international standard for product safety. this document specifies terminology, principles and a process for risk management of medical devices,. This guide covers the definition, importance, principles, and requirements of risk. Medical Device Risk Management.
From exoaernau.blob.core.windows.net
Medical Device Risk Management Documents at Terrence Knapp blog Medical Device Risk Management learn the basic terms, concepts and methods of risk management for medical devices from the us food and drug. learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and. this document provides guidance on the development, implementation and maintenance of a risk management. this document specifies. Medical Device Risk Management.
From www.presentationeze.com
Medical Devices Risk Management PlanningPresentationEZE Medical Device Risk Management learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and. learn the basic terms, concepts and methods of risk management for medical devices from the us food and drug. this document provides guidance on the development, implementation and maintenance of a risk management. this document specifies. Medical Device Risk Management.
From www.asphalion.com
Fundamentals for medical device Risk Management Asphalion Medical Device Risk Management learn the basic terms, concepts and methods of risk management for medical devices from the us food and drug. This transcript covers basic terms,. this document specifies terminology, principles and a process for risk management of medical devices,. This guide covers the definition, importance, principles, and requirements of risk management for medical devices. learn the foundational knowledge. Medical Device Risk Management.
From www.greenlight.guru
Understanding ISO 14971 Medical Device Risk Management Medical Device Risk Management this document provides guidance on the development, implementation and maintenance of a risk management. learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control, and. this document specifies terminology, principles and a process for risk management of medical devices,. learn the basic terms, concepts and methods of. Medical Device Risk Management.
From www.jamasoftware.com
What is Medical Device Risk Management? Blog Medical Device Risk Management this document provides guidance on the development, implementation and maintenance of a risk management. learn the basic terms, concepts and methods of risk management for medical devices from the us food and drug. learn how to perform risk management for medical devices according to iso 14971, the international standard for product safety. learn why, when, and. Medical Device Risk Management.
From www.orielstat.com
ISO 149712019 Basics of Medical Device Risk Management Medical Device Risk Management learn how to perform risk management for medical devices according to iso 14971, the international standard for product safety. this document provides guidance on the development, implementation and maintenance of a risk management. This transcript covers basic terms,. learn why, when, and how to conduct risk management activities for medical devices, such as risk analysis, risk control,. Medical Device Risk Management.